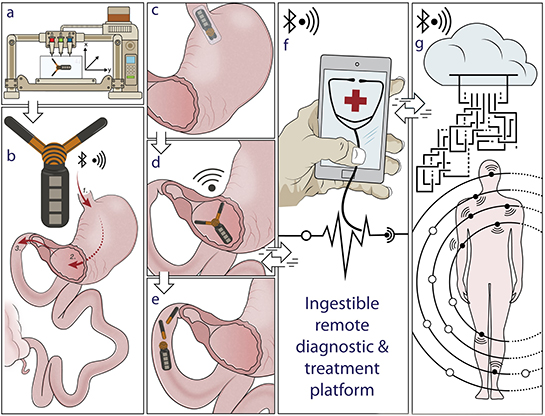
Multimaterials 3-D printing is a highly versatile manufacturing technology that can create unique multicomponent architectures and functional devices, which cannot be fabricated with conventional manufacturing techniques. Recently, researchers have demonstrated the ability to create customized ingestible electronics with multimaterials 3D printing, where the gastric residence period can be potentially tailored based on a specific medical application, which could lead to a personalized diagnostic and treatment that is widely accessible. Pictured is an X-ray image showing the deployed gastric resident electronics (GRE) in a porcine stomach.
Long‐term implantation of biomedical electronics into the human body enables advanced diagnostic and therapeutic functionalities. However, most long‐term resident electronics devices require invasive procedures for implantation as well as a specialized receiver for communication. Here, a gastric resident electronic (GRE) system that leverages the anatomical space offered by the gastric environment to enable residence of an orally delivered platform of such devices within the human body is presented. The GRE is capable of directly interfacing with portable consumer personal electronics through Bluetooth, a widely adopted wireless protocol.
In contrast to the passive day‐long gastric residence achieved with prior ingestible electronics, advancement in multimaterial prototyping enables the GRE to reside in the hostile gastric environment for a maximum of 36 days and maintain up to 15 days of wireless electronics communications, as evidenced by the studies in a porcine model. Indeed, the synergistic integration of reconfigurable gastric‐residence structure, drug release modules, and wireless electronics could ultimately enable the next‐generation remote diagnostic and automated therapeutic strategies.
This research was published today in the latest issue of the Advanced Materials Technologies, a new journal that focuses on technology-related research on materials applications. Assistant professor Yong Lin Kong from the Department of Mechanical Engineering at the U is the lead author of the work, which was started when he was a post-doctoral associate at the Massachusetts Institute of Technology (MIT), working with professor Robert Langer and a gastroenterologist, professor Giovanni Traverso. It was funded in part by the Bill and Melinda Gates Foundation (Grant No. OPP1139921), a grant from the Charles Stark Draper Laboratory, and NIH grant No. EB000244 to MIT, and completed with a partial funding support from the Department of Mechanical Engineering of the University of Utah.
The project involves students, technicians, engineers, and clinician from the MIT, Brigham and Women’s Hospital, Charles Stark Draper Laboratory, Boston University School of Medicine and the University of Utah. Students from the University of Utah who are involved in the completion of the project are graduate student Palal Ghosh, who is a co-author of the paper, and an undergraduate student Hakimi Nazlie. For more information on Kong’s research, publications and future work on 3D printing technologies as well as for exploring opportunities to join the research group, please visit the group’s website.